Displaying items by tag: afr
Following on from our other diagnosis articles, in this article Jon Mitchell is going to talk a little about exhaust emissions.
As we go crawling inside the exhaust of a Porsche, we will take a look at the exhaust emissions, what each of the gasses are, what effect they have on your engine and how they effect the environment.
Exhaust Emissions.
The normal air that we breathe is 80% Nitrogen and 20% Oxygen, with a varied trace of other gasses.
Petrol is a hydrocarbon fuel, comprised of a mixture of carbons and hydrogen. If our engines were 100% efficient, burning all the fuel completely, the oxygen would combine with carbon to form carbon dioxide (C02) and with hydrogen to form water (H2O). The perfect engine therefore would inhale a perfect mixture of air and fuel and exhaust carbon dioxide and water. For every gallon of fuel burnt, a gallon of water would be produced and once the engine was warmed up, the water would exhaust as steam.
Unfortunately a perfect engine does not yet exist and combustion is always to an extent incomplete, in reality the engine in your Porsche will consume close to ideal ratios of fuel and air but will exhaust various compounds including hydrocarbons, carbon dioxide, carbon monoxide, oxides of nitrogen, water vapor and even oxygen.
Most of these gasses are relatively harmless in the quantities that are exhausted by a single Porsche in an open space. In an enclosed space some of them become deadly. However when millions of cars around the world are exhausting these compounds the accumulated effect is harmful to the environment.
So lets take a look at some of these gasses in more detail.
 Carbon Dioxide
Carbon Dioxide
One molecule of CO2 contains one atom of carbon and two atoms of oxygen. CO2 is chemically stable and does not easily react with other substances. It is not poisonous, it is produced by all animals that breathe, including fish. Oxygen is inhaled and Co2 exhaled at a concentration of around 5%.
CO2 is absorbed by all green plants by a process of photo-synthesis which only happens during daylight hours, which also produces and releases oxygen.
A combination of the loss of the rainforest around the world, along with the increased production of CO2 by population, cars and industry, is a concern as CO2 helps trap heat within the atmosphere.
CO2 is a byproduct caused by combusion.
Within an engine the levels of CO2 is likely to be between 13 and 15% with a correctly running engine with a correct air fuel ratio. Less than 8% would indicate an improper air fuel mixture, a missfire or leaking exhaust. Above idle speeds CO2 is likely to increase by around 2% because of the increased RPM causing higher efficiency.
 Carbon Monoxide
Carbon Monoxide
Carbon Monoxide or CO is a very poisonous substance and accounts for around 47% of all air pollution.
CO is also an exhaust emission that is tested for during a UK MOT test. Elevated levels of CO will cause an MOT failure and the levels set by VOSA (The official body of the MOT Test) varies from car to car, dependent on the manufacture date of the car, as well as what equipment is installed.
CO can be deadly to people, if breathed for a sustained period it joins to red blood cells, blocking the path for oxygen molecules, eventually causing death.
CO within the exhaust would indicate incompletely burnt fuel. There will always be a component of CO within an exhaust
High levels of CO are normally caused by to high a fuel quantity being consumed for the amount of air combined with it. This could be caused by a blocked breather, clogged air filter, high fuel pressure a worn camshaft, faulty air flow metering or faulty exhaust gas sensing.
 Hydrocarbons.
Hydrocarbons.
Petrol is an almost pure hydrocarbon, another name for hydrocarbons would be its chemical symbol which is HC.
Hydrocarbons within the exhaust indicate unburnt fuel and are measured in ppm or parts per million.
Hydrocarbons as you can imagine can be raw fuel that is atomised or vapourised, as well as being chemically altered ingredients of the original fuel. They can be extremely dangerous to the eyes, nose and lungs.
Hydrocarbons when combined with high NOx (Oxides of Nitrogen) and sunshine, cause photochemical smog to form in the upper and lower atmospheres. Not such a problem in the UK, but in California it has been a massive problem for many years.
Lead
Lead used to be used in fuels as it lubricated valves, prevented detonation.
Lead is a celular poison for blood, bone marrow and nerve cells. Lead has not been available in mainstream fuels for some time, and even when it was last available in forecourt fuels it was in minute quantities compared with the 1960's and 1970's.
All Porsche models made since the 1960's can run without lead in the fuel in standard specification. Typically cars with cast iron heads require conversion and all Porsche models have aluminium heads, which already have hard valve seats and valve guides not requiring lead in the fuel.
 Oxygen
Oxygen
Oxygen, or O2 (its chemical symbol) consists of two Oxygen molecules and is measured in % of volume.
A very small percentage of O2 will be within the exhaust of a correctly fueled and performing car, as combusion will never be complete and too little oxygen would cause higher hydrocarbons or CO. Typically O2 levels will be around 1 - 2% by volume, pre catalytic converter.
If the O2 level is higher than this, typically it may mean that there is an exhaust leak or the mixture is too lean (may also have high hydrocarbons in some cases).
Oxides of Notrogen or Nitrogen Monoxide and Nitrogen Dioxide, Nitrogen Nitrate.
NOx as it is also known is formed through high pressures and high temperatures.
The N symbolises one Nitrogen atom combined with oxygen atoms (the O), the x symbolises the number of oxygen atoms combined to a nitrogen atom.
NO is Nitrogen Monoxide. Which is one Nitrogen molecule linked to one oxygen molecule. Another name for this is Nitric Oxide. It is caused by by combustion temperatures rising above 1370 degree c.
Typically Nitrogen monoxide, especially at higher engine speeds and loads, causes the production of Nitrogen Dioxide (NO2), Nitrogen Nitrate (NO3) and Ozone (O3). Nitrogen dioxide often forms a brown fog, which is the typical smog that Californians complain about.
One annoyance is that NOX emissions can be caused by high performance engines, especially at their peak efficiency with at cruising speeds with a perfect air fuel ratio (AFR). A way of reducing NOX is to recirculate a small amount of exhaust gasses into the combustion chamber, which reduces combustion temperatures and therefore NOX emissions. However, it also reduces power, so is often employed only in cruising conditions or in conditions tested by government bodies.
Solutions for the engine designer.
Now that we have dealt with the exhaust emissions exhausting directly from the engine, we can cover some ideas and methods of reducing emissions employed by modern engines.
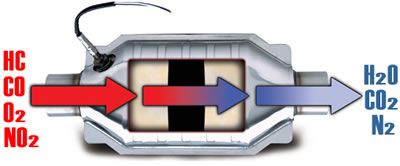 Catalytic converters
Catalytic converters
All modern cars are fitted with a catalytic converter. Also known as a Catalyst or Cat.
Since January of 1993 all UK cars have been fitted with a catalytic converter.
The principle reason for the use of catalytic converters was to complete the combustion of the exhaust gasses so that they are less polluting to the environment.
A Catalyst is something which promotes a chemical reaction without itself being effected. On a car it works as a secondary combustion chamber to complete the combustion of anything left over after the primary combustion has taken place.
At 300 degrees C the catalytic converter begins to work, its happiest temperature is between 400 and 800 degrees, where it will be most efficient, once the catalytic converter passes 1000 degrees, some of the precious metals within the catalytic converter will begin to break down and melt.
When working in the correct temperature range, with the correct levels of exhaust gasses entering it, the catalytic converter will convert CO and HC into H2O and CO2. NOx is reduced by a process of reduction where oxygen and nitrogen are forced apart. The Oxygen combines with CO to produce CO2 and N2.
A weak mixture with a high level of O2 is good for the oxidation of CO and HC. On the other hand, a rich mixture with some CO aids the reduction of NOx. A comprimise is reached by the engine running at a mixture of 14 to 1. This means the engine is slightly richer than ideal and use more fuel, but it will reduce NOx emissions, yet will be lean enough to combine CO and HC to create H2O while also running the catalytic converter hot enough to stay within its ideal temperature band. Often with modern engine management the engine will quickly alternate between slightly rich and slightly lean to allow the catalytic process to get the best of both ideals.
H2S is a gas not tested in the UK for MOT testing, but is often released by a new catalytic converter, which causes the smell of rotten eggs that can sometimes be smelt with a new cat. However sometimes with a car running rich will also cause this smell on the overrun as it becomes lean and the catalytic converter creates this gas as a byproduct of burning off excess carbon caused by the rich state.
A car fitted with a catalytic converter has to run perfectly at all times, a misfire, sensor fault, wrong fuel pressure, or many other, can permanently damage the catalytic converter. As an expensive item it is wise to follow a specialists advice with preventative work on the engine, before it becomes a fault, but also if you experience a fault, to have it attended to immediately.
Lambda
As the engine operates, air and fuel are mixed together, drawn into each cylinder. The Air Fuel Ratio (or AFR) at which air and fuel burn at their most efficient is known as the Stoichiometric point and is where HC and CO should be at their lowest and CO2 should be at its highest. This ratio is 14.7:1 by weight, and it is also called Lambda = 1 which is the greek word for "correct".
Various fractions of Lambda can can be converted into AFR by multiplying 14.7 by the fraction. The AFR/Lambda chart is the result.
Although lambda=1 is not the ideal point fuel consumption, it is the best comprimise for using a catalytic converter to oxidise CO, HC and NOx. Therefore if the Lambda can be kept between 0.98 and 1.02 the efficiency of the catalytic converter will be in excess of 90%. The reason being that the lower emissions from the engine reduce the work required for the catalytic converter to convert these harmful gasses into more acceptable ones.
 Oxygen Sensor
Oxygen Sensor
A sensor placed within the exhaust system to sense the levels of oxygen present following combustion, the Oxygen Sensor is known by many names. Including the Oxygen Sensor, Lambda Sensor, Exhaust Gas Oxygen Sensor (EGOS) or even when used with a heating element a Heated Exhaust Gas Oxygen Sensor (HEGOS). For the purpose of this article, we will stick with calling it the Oxygen Sensor.
The sensor typically has a porous ceramic tip, this tip often contains two platinum electrodes, the outer surface electrode is exposed to exhaust gas, with the inner electrode exposed to atmospheric air. The difference in oxygen content between the two electrodes produces an electrical voltage, which is read by the engine management computer to analyse the oxygen remaining in the exhaust after combustion.
The sensor is often placed before the catalytic converter for the engine management system to evaluate and adjust the mixture being fed to the engine, however more modern cars now also have one after the catalytic converter, which allows the engine management system to know how efficiently the catalytic converter is performing.
The voltage generated by an oxygen sensor is inversely proportional to the difference in oxygen content seen in the exhaust compared to the oxygen in the ambient air around the car. The engine management system will use this signal to increase or decrease the fuel provided to the engine on a split second by second basis, in effect allowing the engine to be self tuning.
Typically voltage being seen by an engine management system would be between 0.1v (lean) and 1.0v (rich). If you were to connect a volt meter to the lambda sensor while the engine is running, you would likely see the voltage changing from above and bellow 0.55volts on a regular basis, possibly to quickly for the volt meter to be able to display, in which case an average voltage of 0.55 volts may be observed.
Analysing the quantity of oxygen remaining after combustion is an excellent indicator of how rich or lean the mixture of fuel is being supplied to the engine. In theory an engine management system could solely use an oxygen sensor to decide on the quantity of fuel to inject into the engine, keeping the exhaust gasses constantly at the correct oxygen content indicating a proper mixture, which in turn would extend the lifespan of the catalytic converter and the oxygen sensor, while also providing a reasonable fuel economy. However, a car requires different levels of fuel for different driving conditions and demans, such as accelloration or decelloration, on full throuttle or part throttle, if the mixture remained at 14.7 to 1 at all times, the car would drive very poorly. As such, engine managements tend to only take notice of the oxygen sensor when cruising at a constant or near constant speed.
More recently a new type of oxygen has been available, known as a wideband sensor, which is able to read a wider range of air fuel ratios, which an engine management system can monitor and compare with target air fuel ratios at a wide range of demands and conditions to make alterations to fueling. These are also used by enthusiasts and tuners so they can monitor their exhaust oxygen content as a warning instrument as well as a tuning device.
An oxygen sensor can however be fooled. A perfect air fuel ratio may for one reason or another not fully combust both the fuel and air mixture, the oxygen sensor will see this surplus fuel, which may be interpreted as a weak mixture, especially dangerous if using an oxygen sensor to tune a car.
 Mass airflow sensor (MAF Sensor)
Mass airflow sensor (MAF Sensor)
A mass airflow sensor (also known as a MAF sensor) is a sensor sometimes used by an engine management system to measure the mass of air being consumed by the engine at any one moment in time.
As the fuel pressure and the flow from fuel injectors is usually predictable as to how much fuel will flow through them per second, knowing the correct amount of air entering an engine can provide a good assumption of how long to pulse the injectors open for, to provide the correct air fuel ratio for any one given engine speed or loading. In systems with a MAF sensor, often an oxygen sensor is also used, especially in catalytic converter equipped vehicles to provide perfect fueling in all conditions. However a faulty MAF sensor may cause the engine management system to believe there is a fault with the oxygen sensor, or indeed the other way round.
Air flow meter - Also known as a Volumetric Air Flow Meter (VAF Sensor)
Before MAF sensors were invented, the common way to measure fuel entering a fuel injected car was by using a Volumetric Air Flow sensor. When coupled with a temperature sensor, a VAF sensor can make a good approximation of the volume and mass of air entering an engine. However, their design, owning to a spring loaded trap door connected to a sensor causes this to be a component which is not only subject to wear, but also an item which offers resistance to air entering an engine, lowering performance.
Manifold Absolute Pressure Sensor - Also known as a MAP sensor
A map sensor measures the air pressure seen at the intake manifold, as the amount of air which can pass a throttle butterfly at any opening position is fixed, the combination of MAP sensor with a throttle position sensor, can also make a good aproximation of the volume of air being consumed. When combined with a temperature sensor, like with a VAF sensor, the engine is able to calculate the mass of air being consumed and calculate the fuel required for any given demand placed on the engine.
Turbo charged vehicles sometimes are fitted with both a MAF and MAP sensor to provide increased accuracy as well as telling the engine management system how much boost is being provided by the turbo or turbos. This is sometimes essential as the requirement for fuel alters with the pressure it is being compressed to. Typically, higher intake pressures require an increase in fuel quantity to avoid damage to the engine and provide maximum efficiency.
Engine Coolant Temperature sensors
Engine coolant temperature sensors are essential to the smooth and safe running of an engine. When an engine is cold, the requirement for fuel is higher than when one is warm. In addition a catalytic converter will not work until it has reached a temperature of above 250 degrees C, which means as well as an engine management system using the engine temperature to increase or decrease fuel quantity, it will also use it to know when and when not to take notice of the oxygen sensor.
Closed Loop or Open loop.
When the conditions are right, an engine management system will begin to rely on a oxygen sensor to calculate and govern the fuel quantity provided for the engine. When this is the case the condition is known as closed loop. Typically this will be when the engine has reached opperational temperature, the throttle is neither at wide open throttle nor closed at an idle position. When these conditions are not all made, the engine management system will be known as running in open loop mode.
Engine Management System (EMS), Electronic Control Unit (ECU), Digital Motor Electronic (DME)
There are many names used for the electronics used to control and engine management system as shown in the title of this section. However for the sake of this article we have referred to the Engine Management System or EMS. However all the names mean the same thing, a sophisticated (for its day) computerised control unit which monitors several sensors, decides how much fuel to supply, when to supply an ignition source (spark) along with a few housekeeping tasks and sometimes recording faults found. One of the important jobs is to ensure the car runs efficiently and with the minimum of exhaust gas emissions.
Faulty Engine
An engine fault can cause problems with exhaust emissions. A good example would be wear causing a loss of compression, which may cause the assumptions of the engine management system to be wrong and therefore the wrong quantity of fuel provided, which can result in high levels of CO and possibly hydrocarbons. In addition, and engine which burns oil can sometimes cause a raised level of hydrocarbons, which is because like the fuel you intend to use, the engine oil is a complex carbon and fuel source, which when incompletely burnt, will cause hydrocarbons and in some cases raised CO levels depending on how well it is being combusted.
Exhaust System
A faulty exhaust system may have a leak. Often you will hear people refer to an exhaust blow, but not so many people know that this will also cause air to enter the system.
For every combustion event there will be a high pressure wave traveling along the length of the exhaust, causing some exhaust gasses to possibly escape before they have reached the tailpipe. However behind each one of these high pressure waves there will also be a lower than ambient negative pressure wave, which will suck ambient air into the exhaust.
If the exhaust leak is before the oxygen sensor, the sensor will see this additional air entering the exhaust as a lean mixture, possibly causing the engine management computer to increase the mixture provided to the engine.
If the exhaust leak is after the oxygen sensor, the sensor will be oblivious to the additional air, but any diagnostic work performed by measuring the exhaust gasses at the tailpipe will be false, in particular the oxygen level.
The MOT Test
Typically, the MOT test in the UK is interested in CO, HC and O2 levels in the exhaust system, elevated quantities of any of these, beyond pre set limits will cause a failure of the UK MOT test.
 Diagnosis - Hydrocarbons.
Diagnosis - Hydrocarbons.
Typically, hydrocarbons are caused by an incomplete burn of the fuel provided to the engine. Normal causes of this will be poor ignition, improperly atomized fuel, consumption of engine oil or an incorrect mixture. As such, diagnosis needs to focus on engine health, ignition, fuel system health and other exhaust gasses which are present.
With hydrocarbons many garages will try to say it is a mixture problem, however unless the mixture is extremely over lean, hydrocarbons will more often be caused by something stopping that cylinder from firing correctly, leaving fuel left over. Often a rich mixture will fire correctly, however will result in high CO levels.
So if you see normal CO levels but high hydrocarbons, then the problem is probably a ignition or engine fault.
If you see low CO levels (lower than reasonable) and high hydrocarbons, the problem is probably one of the mixtures being too lean, so look into that.
If the CO levels are very high and you also have elevated hydrocarbons, the problem may well be the high mixture levels (too rich)
Overall, Hydrocarbons are one of the most important exhaust emissions to help point at the cause of an issue, if the hydrocarbons are low, it normally indicates a good ignition system as well as a fit engine.
 Diagnosis - Carbon monoxide
Diagnosis - Carbon monoxide
Carbon monoxide levels are usually caused by a rich fuel mixture, which can be caused by faulty air measurement, high fuel pressure or bad exhaust oxygen sensing. However it sometimes can be a result of poor air filter condition or on older cars of a need to adjust a carburetor.
Carbon monoxide is nearly always caused by too much fuel being consumed by the engine, which can be caused by a fault in the electronic engine management system, or with the carburettor on a older engine.
It can also be caused by a faulty injector, fuel pressure regulator or o2 (lambda) sensor.
High carbon monoxide or dioxide can be a killer, so while trying to diagnose these faults, it is really important to do so in a well ventilated area, as these gasses can be a silent killer with no odor or other side effects other than a headache and death!!
Carbon monoxide levels can also be high if the engine is cold, so make sure your engine is fully warmed up before you pay too much attention to the levels, and remember that it takes twice as long for the engine to be completely warmed up than it takes for the coolant to be up to temperature. This is because water absorbs heat much faster than aluminium, iron, steel or engine oil... so be patient.
 Diagnosis - Oxygen
Diagnosis - Oxygen
High levels of oxygen within the exhaust system can be an indication of poor exhaust sealing. But it can also be a sign of poor combustion, depending on what other exhaust gasses are present, it can indicate poor ignition, fuel system health or engine health.
It is also worth bearing in mind that if your exhaust system is not in good condition, air can be drawn into holes in an exhaust, just as much as a poor exhaust can blow its exhaust gasses out of gaps (a surprise to most people) which can cause elevated o2 levels in the exhaust gas.
The other problem with high o2 levels is that they can cause the o2 (lambda) sensor to see this air as a weak mixture, which in turn can cause the engine management system to add lots of excessive fuel to be added, which will show up as high carbon monoxide readings and possibly even high hydrocarbon levels. This means that if one of your emissions which seem high is the o2 level, please check the condition of your exhaust system and make sure that except for the tailpipe it is extremely air tight.
Good garages like JMG Porsche will use a smoke tester to test to see if the exhaust system is airtight (where it should be) as well as using diagnostic skill to find the cause of the high o2 levels and knowing what other elements may effect them.
Diagnosis - Drivability
Often a fault can manifest itself as poor drivability of the car. In these cases, exhaust gas analysis can assist in tracking down the fault.
Symptms and causes.
An important note to make before closing this article, is that there is a very large difference between symptoms and causes.
A good example might be an engine driving poorly, the exhaust emissions for carbon monoxide may be high and the engine management system may indicate that it believes the oxygen sensor is at fault. However both these items would be symptoms, even if when tested the oxygen sensor is found to be faulty. The actual cause may be something else, such as a mass airflow sensor providing a false reading of more air entering the engine than is actually happening. In this case the cause may have actually caused the oxygen sensor to become faulty, which will result in more than one faulty component, two in this case, one being a symptom, the other being the cause.
With this in mind, it is important to never assume the cause of a fault has been found, otherwise, as with the above example, the same fault will re-occur with a new component becoming faulty again. In all cases, even if a test shows a part to be faulty, further tests should be made to other components to be sure there are not other symtoms, faulty components and indeed one or more faulty components which are actually causes of the other symptoms.
It is therefore often important to allow your technician to perform multiple tests before and after a repair is made, it is often not the technician performing needless tests, but in fact ensuring that both the symptom and the causes have been eliminated, otherwise an expensive component may need to be changed more than once!




